THE GUARDIAN™ CONNECT SYSTEM.
Available for OS Devices.
Now FDA approved with AndroidTM devices. Available this summer.
Call Today 1-800-308-0198

WHAT IS SMART CGM?
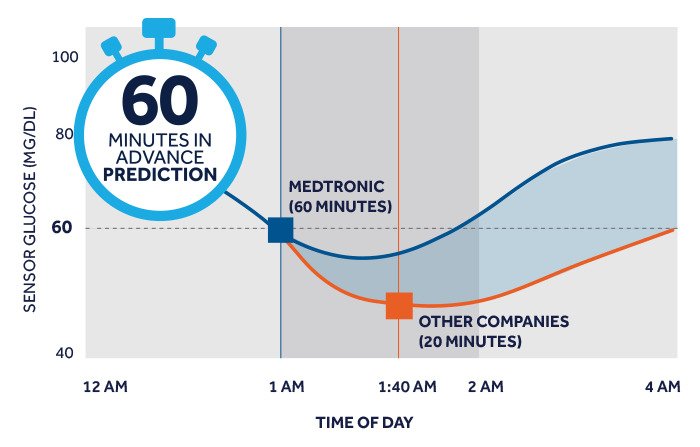
Smart CGM predicts future high and low sensor glucose events up to 60 minutes in advance and provides access to additional algorithms and insights that can inform you of clinically relevant glucose patterns.†
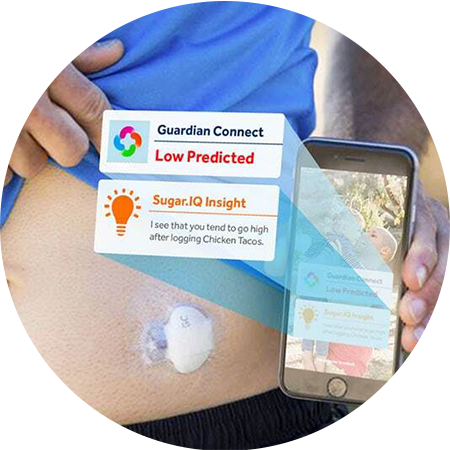
ACCURATE
Measures sensor glucose 24/7 and sends alerts when a high or low is detected.
DISCREET
Discreetly view your glucose reading on your smart phone. Get notifications on your smart watch.
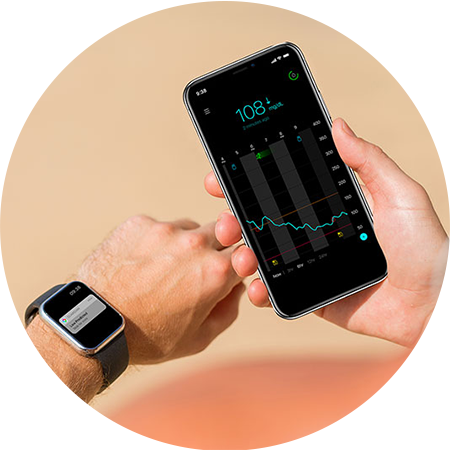
INFORMATIVE
Trend arrows provide insight on the direction your glucose is heading, customizable predictive alerts help you take action earlier.
REAL-TIME INFORMATION FOR ON THE SPOT DECISIONS
You decide in advance when you want to be alerted between 10-60 minutes in 5 minute increments. Minimum two calibrations per day required.
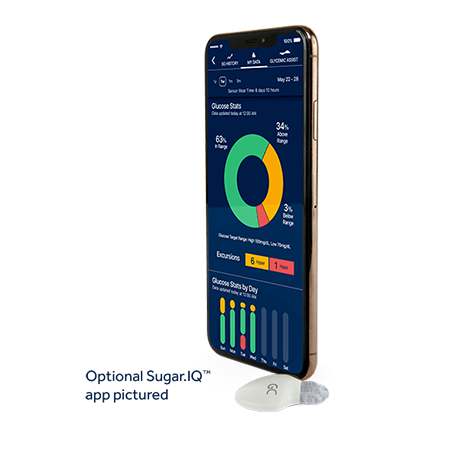
EXCLUSIVELY AVAILABLE WITH THE GUARDIAN™ CONNECT SYSTEM IS THE SUGAR.IQTM DIABETES ASSISTANT APP.
Available for devices.
The Sugar.IQ™ assistant supercharges the Guardian™ Connect system’s smarts by giving you a big picture view of your glucose patterns – and the factors that affect them.
WATCH THE GUARDIAN™ CONNECT SYSTEM VIDEOS
SEE THE SAME REPORTS AS YOUR DOCTOR - AND
COLLABORATE ON YOUR CARE.
Anytime access to glucose levels and auto uploads to the CareLink™ Connect platform.
PEACE OF MIND
FOR CARE PARTNERS.
Stay connected to your family and friend with automatic text message alerts through a unique care partner app.
WHAT TO EXPECT
COVERAGE
We check your insurance and pharmacy benefits for coverage. You choose the one that is best for you.
24-HOUR TECHNICAL SUPPORT
We have your back. Our 24-hour Technical Support is staffed 24/7.
PERSONALIZED SUPPORT
Our team of dedicated clinicians will help guide you through training and ongoing support, our goal is that you StartRight™.
1-YEAR WARRANTY
Peace of mind: we’ll replace your device. See conditions.
30-DAY RETURNS
If you are not happy, you may be able to return your product. Please contact our Returns Department for more information.
† Smart CGM predicts future high and low sensor glucose events up to 60 minutes in advance and provides access to Sugar.IQ™ insights that can inform users of clinically relevant glucose patterns.
‡ Android is a trademark of Google LLC.
Important Safety Information: Guardian™ Connect CGM System
The Guardian™ Connect system requires a prescription and is indicated for continuous or periodic monitoring of glucose levels in the interstitial fluid under the skin, in patients (14 to 75 years of age) with diabetes mellitus. The system is intended to complement, not replace, information obtained from standard blood glucose monitoring devices, and is not recommended for people who are unwilling or unable to perform a minimum of two meter blood glucose tests per day, or for people who are unable or unwilling to maintain contact with their healthcare professional. The system requires a functioning mobile electronic device with correct settings. If the mobile device is not set up or used correctly, you may not receive sensor glucose information or alerts. For complete details of the system and its components, including warnings, contraindications, and precautions, please consult the user guide at https://www.medtronicdiabetes.com/download-library and important safety information.
Important Safety Information: Sugar.IQ™ App
The Sugar.IQ™ app (MMT-8100) helps manage diabetes by facilitating the logging and display of meal entries and sensor glucose (SG) data, tracking meal log entries, reporting insights of how meals affect glucose levels, and supporting good choices and trends with motivational messages. The app serves as an additional display for real-time CGM data from the Guardian™ Connect system through the CareLink™ Personal software. It is not intended to provide medical advice and should not be relied upon for such purpose. The app is not intended to replace the real-time display of the CGM data on the Guardian™ Connect app, control any functions of the connecting device, calculate insulin or other drug doses, or modify date or control functions of the Guardian™ Connect system. All therapy decisions should be made by the app user based on blood glucose (BG) measurements obtained from a BG meter. Changes to treatment should only be made in consultation with a healthcare professional (HCP). For complete details, consult the user guide at https://www.medtronicdiabetes.com/download-library and important safety information.
The system is intended to complement, not replace, information obtained from standard blood glucose monitoring devices. All therapy adjustments should be based on measurements obtained from standard blood glucose monitoring devices and not on values provided by the system.
Important Safety Information: CareLink™ Software
The CareLink™ software is intended for use as a tool to help manage diabetes. The purpose of the software is to take information transmitted from insulin pumps, glucose meters and continuous glucose monitoring systems, and turn it into CareLink™ reports. The reports provide information that can be used to identify trends and track daily activities—such as carbohydrates consumed, meal times, insulin delivery, and glucose readings. NOTE: CareLink™ report data is intended for use as an adjunct in the management of diabetes only and NOT intended to be relied upon by itself. Patients should consult their healthcare providers familiar with the management of diabetes prior to making changes in treatment. For more details, please consult important safety information. and the appropriate CareLink™ User Guide at https://www.medtronicdiabetes.com/download-library.
Important Safety Information: MiniMed™ 670G System
The Medtronic MiniMed™ 670G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons, seven years of age and older, requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 670G system includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on Continuous Glucose Monitor sensor glucose values and can suspend delivery of insulin when the sensor glucose value falls below or is predicted to fall below predefined threshold values. The system requires a prescription. The Guardian™ Sensor (3) glucose values are not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. A confirmatory finger stick test via the CONTOUR®NEXT LINK 2.4 blood glucose meter is required prior to making adjustments to diabetes therapy. All therapy adjustments should be based on measurements obtained using the CONTOUR®NEXT LINK 2.4 blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the CONTOUR®NEXT LINK 2.4 blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an Alternative Site (palm) or from a control solution test. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode.
WARNING: Medtronic performed an evaluation of the MiniMed™ 670G system and determined that it may not be safe for use in children under the age of 7 because of the way that the system is designed and the daily insulin requirements. Therefore this device should not be used in anyone under the age of 7 years old. This device should also not be used in patients who require less than a total daily insulin dose of 8 units per day because the device requires a minimum of 8 units per day to operate safely.
Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. The safety of the MiniMed™ 670G system has not been studied in pregnant women. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information#minimed-670g and the appropriate user guide at https://www.medtronicdiabetes.com/download-library
© 2020 Medtronic. All rights reserved. Medtronic, Medtronic logo and Further, Together are trademarks of Medtronic. Third party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company.





